Exclusive: Lab Report Commissioned by WHO Shows DEG Contamination in Cough Syrups

New Delhi: While the Indian drug regulator gave a clean chit to Maiden Pharmaceuticals for the deaths of 69 children in the Gambia, a lab in Geneva that received samples of four cough syrups manufactured by the Haryana-based company from the World Health Organisation (WHO) has identified the presence of the toxin diethylene glycol (DEG).
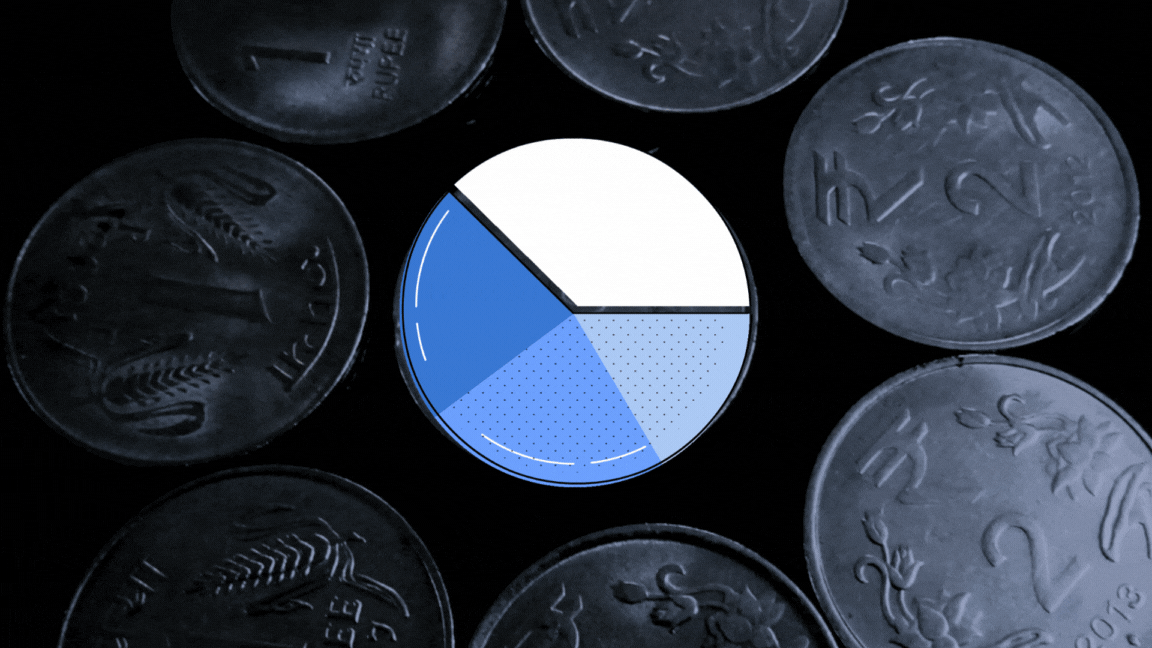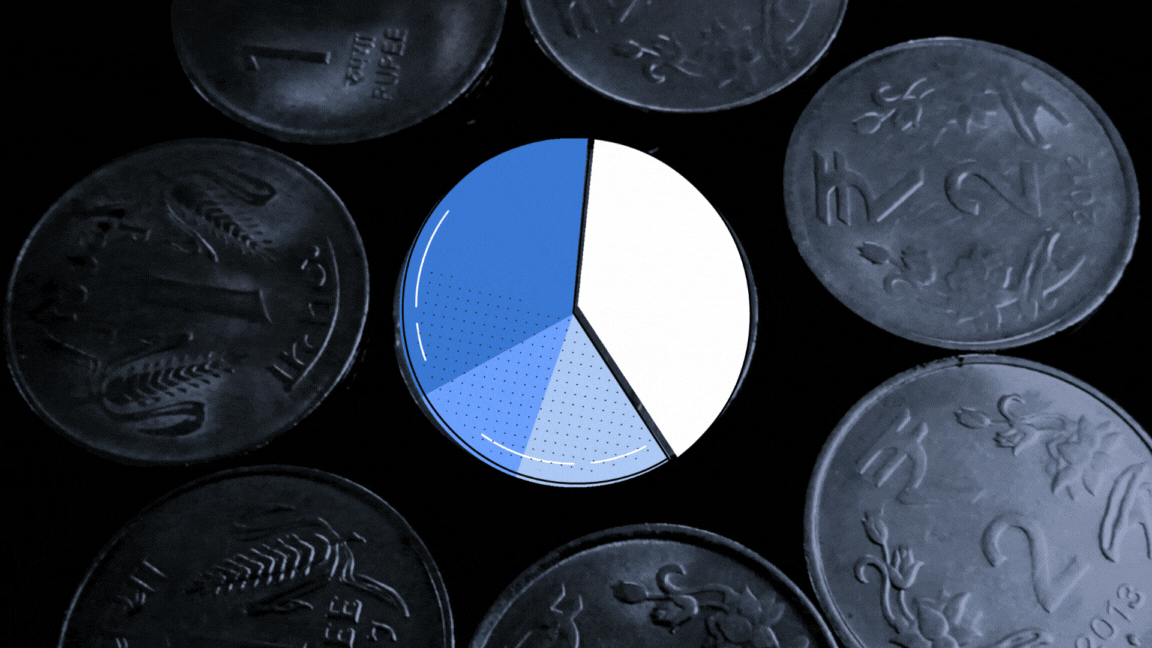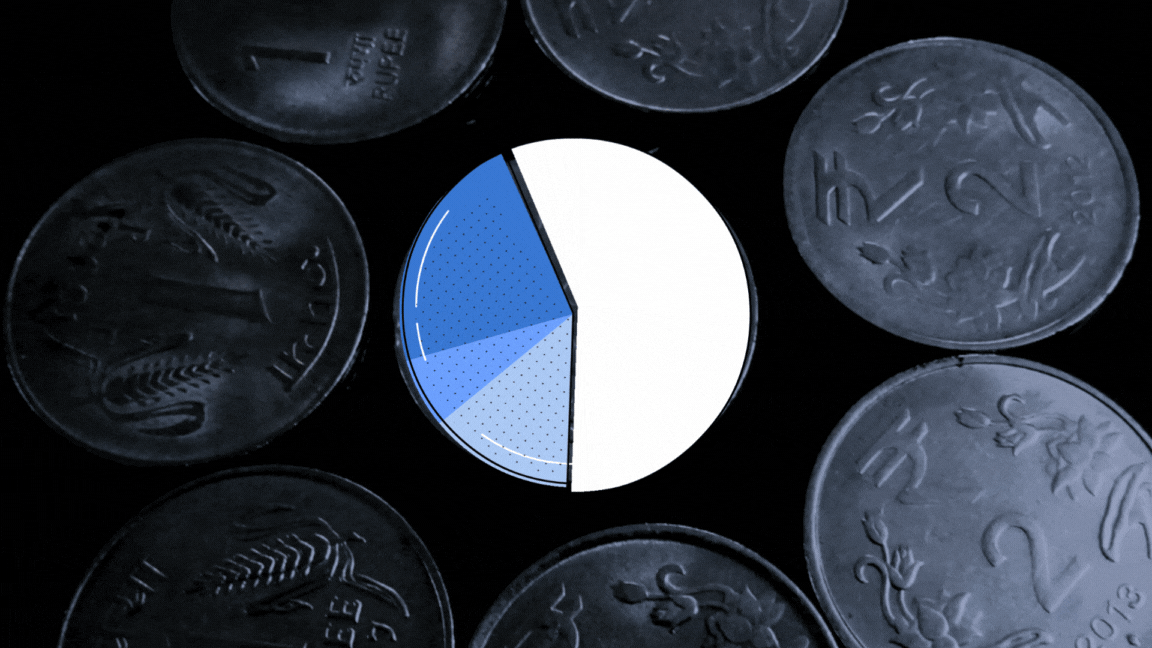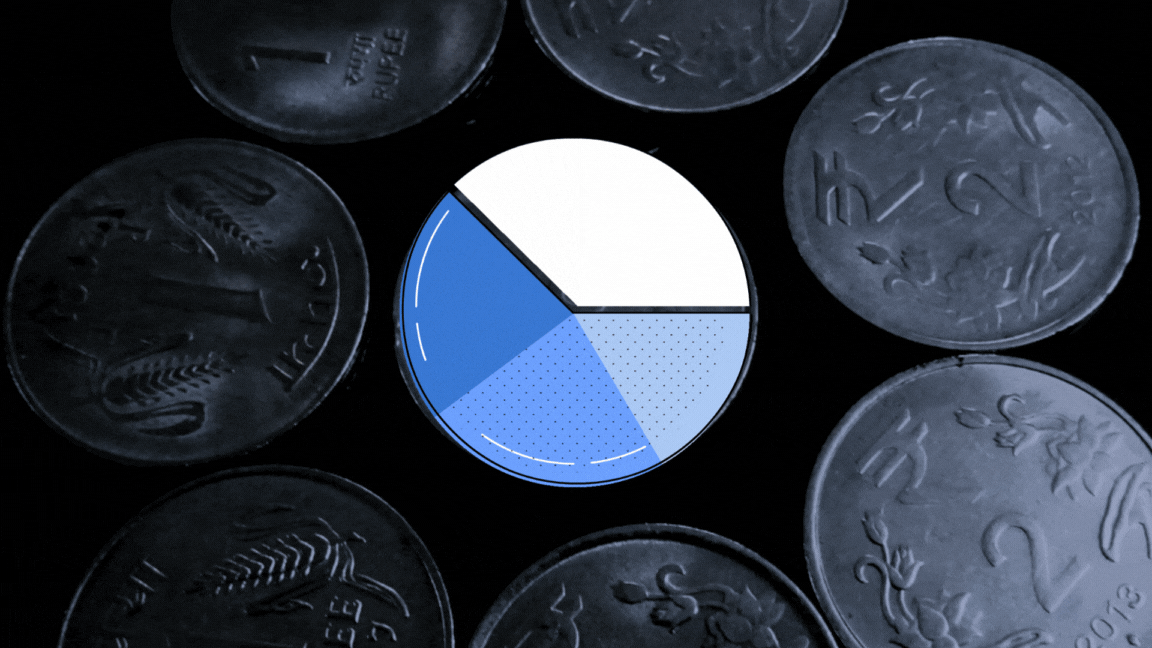
The UN health agency had sent 23 samples of paediatric formulations to the lab to investigate if they were ‘potentially’ linked with the deaths of 69 children in Gambia. The toxin diethylene glycol (DEG) was found to be present between the range of 1.0%-21.30% weight/volume (w/v) in samples of four cough syrups made by Maiden Pharmaceuticals, The Wire has learnt.
The presence of DEG is completely banned in drugs.
The report, dated September 30, 2022, found another toxin, ethylene glycol (EG) present between the range of 0.3%-5.9% w/v in the same four samples.
The Wire has a copy of the lab report.
These results are in sharp contrast with the investigation carried out by India’s drug regulator, the Central Standards Drugs Control Organisation (CDSCO), which claimed that the ‘control samples’ of the four drugs in question had no DEG contamination at all. For every batch of the drug which is released in the market, a manufacturer is supposed to preserve samples, known as ‘control samples’, for any future investigation. The CDSCO has not made the report public.
In an emailed reply given on December 17, the WHO refused to comment on the specifics of the lab report when The Wire shared it with the agency. However, in response to another email The Wire sent on December 16, the WHO confirmed that samples of the cough syrups which were manufactured by Maiden Pharmaceuticals were sent to labs in Switzerland and Ghana.
In a ‘product alert’ issued on October 5, 2022, a few days after the Geneva lab’s report was signed, about the four cough syrups, the WHO said that DEG and EG were found in “unacceptable amounts”.
When The Wire asked the WHO if it would review the product alert in light of the recent findings of the CDSCO, the former gave no such hint. “[The] WHO stands by the action taken,” it reiterated.
A Cochin-based biochemist converted the w/v% concentrations for DEG and EG for all the four samples for The Wire, taking into account the density of DEG as 1.11gm per cubic cm and EG as 1.12 gm per cubic cm. His name is being withheld at his request.
According to his calculations, the v/v% concentration of DEG tested for four samples in the Swiss lab range from 0.9% — 19.02% (for values of each product see graphic below). This was more than what investigations revealed about the Haiti DEG mass poisoning event in which 86 children died in 1996. The DEG concentration in health products connected with this event was found to be 14.4v/v%. In a similar such event, 21 children died in Panama in 2006. The DEG concentration was found to be 8.1 v/v% while it was 19.3 v/v% in the case of Nigeria mass poisoning event that took place in 2009.
The biochemist, while making these calculations, said the results were approximate and could differ by 0.2-0.5% taking some environmental factors into consideration.
DEG is a chemical that is used as a solvent for industrial purposes but not for pharmaceutical purposes. In the manufacture of drugs, propylene glycol (PG) is to be used as a solvent. But either due to lack of oversight or with the purpose of reducing costs, manufacturers procure PG contaminated with DEG. And if the manufacturer fails to test the raw materials before using them, the chances of DEG poisoning are very high.
Both DEG and EG are banned in drugs as they are known to cause renal failure and/or several other adverse health impacts. The WHO’s ‘product alert’ asked all countries to remove these four products from the market and consider them unsafe till analysed. It said, “Toxic effects can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state, and acute kidney injury which may lead to death.”
“The substandard products referenced in this alert are unsafe and their use, especially in children, may result in serious injury or death.”
Gambian epidemiologist says CDSCO report ‘unbelievable’
Meanwhile, Adebayo Peter Adewuyi, a member of the emergency response team instituted by the Gambian government to deal with the outbreak told The Wire that the CDSCO’s conclusions are ‘unbelievable’ to him. In a phone call on December 16, he also confirmed that the samples which were sent by the WHO with the support of the Gambian government for testing in labs, including the ones at the Switzerland lab, had confirmed contamination with DEG and EG.
Adewuyi is an epidemiologist and a member of the African Field Epidemiology Network (AFNET), a non-profit network aiding epidemiological training programmes in Africa. However, he clarified he was speaking in his personal capacity.
He said as soon as the products were withdrawn from the market, there have not been any deaths due to acute kidney injury (AKI) in children. “If you are able to remove the source, the outbreak can be contained. Since we withdrew the syrups, we haven’t had a single death due to AKI in more than 2 months,” he added.
His assertions are backed up by the death toll reported in WHO’s African weekly bulletins. The first deaths were reported in the WHO’s African bulletin for August 8-14. The September 19-25 bulletin gave the first details and described the outbreak thus:
“Based on the available evidence, the event has been classified as acute kidney injury secondary to E. coli infection, compounded by toxins contained in medicines. However further epidemiological and laboratory investigation are urgently required to better identify the aetiology and contributory factors and therefore this is subject to change when more information becomes available.” [Emphasis supplied]
In this bulletin, 75 cases and 50 deaths were reported.
The bulletins show that when Gambia started removing the four cough syrups from its market in October, the deaths and cases also became stagnant.
It is pertinent to mention here that no causal link has been established by the WHO or any other agency between the withdrawal of the alleged substandard cough syrups and the decline in deaths.
Causality assessment
An official of Gambia’s drug controller office, Tijan Jallow, stated last month that his department had not confirmed any link between the four products in question and the AKI deaths in children. He also added that some of the children who died did not consume the syrup.
This enraged patient advocacy groups in the Gambia, who hit the streets to demand that the statement be retracted. They even rejected the ex-gratia offered by the government. The Gambian health ministry, under which the regulator functions, is also under fire, allegedly for not doing enough.
Adewuyi claimed that the statement made by a representative of the Gambian drug controller was unofficial. The Gambia’s director of health services, Mustapha Bittaye, did not respond to The Wire‘s efforts to reach out to him for a comment on the ministry’s stance towards Jallow’s statement. Adewuyi asked this correspondent to wait for the report of the committee of the Gambian parliament, which was formed specifically to investigate the deaths. Asked if he was aware that children had died without taking any of the four syrups, Adewuyi said yes, but added that the investigation had not yet established if those deaths happened due to AKI and were related to the outbreak which started in July.
The CDSCO, meanwhile, had also raked up the issue of causality in the letter that it sent to the WHO on December 13 referring to media reports that had published Jallow’s statement. The letter was officially released to the media by the Indian health ministry.
The Indian regulator had accused the WHO of not doing enough in trying to help it with causality assessment and adopting a “strangely contradictory position” to its earlier iterations that “affirmed its commitment to provide granular details of the incident on causal relation”. The WHO didn’t respond to The Wire‘s query seeking a response to this charge.
Causality assessment is a tough job, the Gambian epidemiologist says. “You may not be able to prove that everybody [who died] consumed the drug but the presence of DEG [in medicines] itself is criminal… When we asked [the parents of children who died] if they had given any of these medicines, some of them would say no because they feared punishment. But eventually, deeper inquiries did reveal in some of those cases that … syrups [were given] to children,” he said.
“This is a Muslim country. You can’t easily ask for bodies of dead children for investigation as they are buried immediately,” he explained.
Nonetheless, he added, that a causality assessment was being carried out by “independent assessors brought in by the WHO and Gambian government.” That report is also expected in a short while, he said.

Maiden Pharmaceuticals Ltd. Photo: Reuters
CDSCO’s charges abound
Meanwhile, the Indian regulator had also charged the WHO with making “premature deductions without waiting for an independent investigation.” It is unclear what it meant by “independent investigation”.
The CDSCO added that by making these deductions, the WHO adversely impacted the reputation of the Indian pharmaceutical industry and caused “irreparable damage to the supply chain of pharmaceutical products.” The WHO did not react to this accusation either in the reply to The Wire.
But in a classic case of the left hand of government not knowing what the right hand is doing, a minister’s statement in parliament contradicted the CDSCO’s claim on alleged damage to the reputation of India’s pharma industry.
Answering a query in the Rajya Sabha on December 13, 2022 if the Gambian tragedy had “impacted the Indian pharma business in the international market”, the minister for chemicals and fertilisers Bhagwanth Khuba replied in the negative. She said:
“There have been reports both in domestic and international media on the incident. There is no observable impact on the Indian Pharma Export in the past few months that can be attributed to this incident [sic].”
The CDSCO’s letter to the WHO, also sent on December 13, said that Haryana’s drug control authorities had pointed towards violations of Good Manufacturing Practises (GMP) at the plant of Maiden Pharmaceuticals. It said the company did not maintain proper records for ‘manufacturing and testing’, for which the firm was issued a show cause notice. But since the CDSCO has not made the report public, there are many unknowns, including if the chain of custody of ‘control samples’ was also compromised in the company’s improper record-keeping exercise.
The Wire‘s attempts to reach Maiden Pharmaceuticals’s director Naresh Goyal were futile.